- Статьи
- Science and technology
- Comprehend the limits: flash drives with a molecule will help to store unattainable amounts of memory
Comprehend the limits: flash drives with a molecule will help to store unattainable amounts of memory

For the first time, scientists have been able to synthesize special rare earth metal complexes that remain stable in the air and in the presence of moisture, as well as when heated. Some of them exhibit the properties of molecular magnets, in which only one molecule can be the information carrier. They can potentially form the basis of new storage media with currently unavailable memory capacity. This, in turn, makes it possible to take the miniaturization of electronic devices to a new level, as well as create new functional materials and sensors. For more information about the technology, see the Izvestia article.
What are molecular magnets?
Modern storage media and memory devices have almost reached the possible limit of miniaturization, therefore, fundamentally new technologies and materials are needed to compactly store large amounts of data. In particular, monomolecular magnets are considered promising — in them only one molecule serves as a carrier of information. Thanks to this, they will allow you to record, store and process data with a density and speed inaccessible to other technologies. However, molecular magnets are not yet used in practice due to their low stability: most of them retain their structure and properties only at extremely low temperatures and are destroyed by exposure to air and moisture.
Researchers from the A.N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences (Moscow) and the G.A. Razuvaev Institute of Organometallic Chemistry of the Russian Academy of Sciences (Nizhny Novgorod) and colleagues synthesized stable organometallic complexes of rare earth metals, some of which demonstrated the properties of molecular magnets in an applied magnetic field.
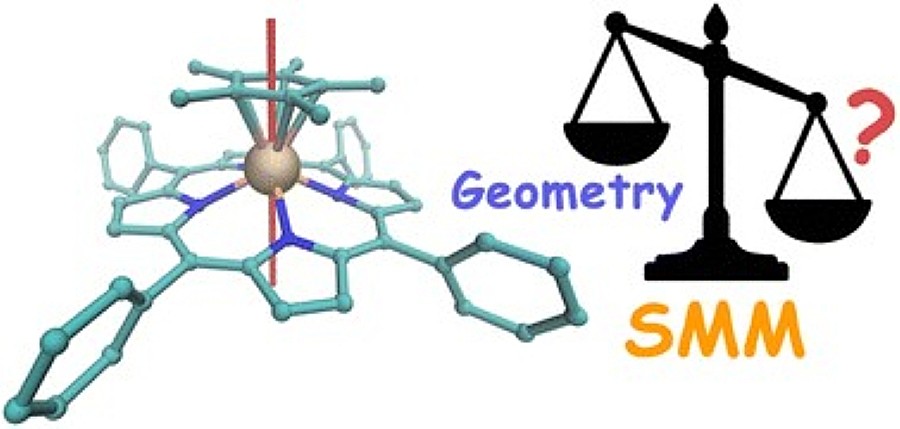
The new compounds have a sandwich structure: a metal ion (yttrium or lanthanides of terbium, dysprosium, holmium and erbium) in them is enclosed between two parallel planar ligands, fragments of organic molecules. Experiments have shown that such complexes are resistant to air and moisture, and are also able to withstand vacuum sublimation (transition to a gaseous state). Due to these properties, they can be used to create new functional materials, composites, and films.
A study of the magnetic properties of the obtained complexes has shown that the formal fulfillment of geometric requirements, such as an axial structure with a parallel arrangement of ligands in the coordination sphere of metal ions, is insufficient to produce the properties of true molecular magnets. Terbium, dysprosium, and erbium complexes exhibit these properties only in an applied magnetic field. The results obtained greatly contribute to understanding how the nature of ligands and the electronic structure of such molecules affect their magnetism, which is important for the design of effective molecular magnets.
— In the future, we plan to expand the range of organometallic compounds of lanthanides of this class and investigate the factors affecting their magnetic properties and stability. It should be noted separately that when switching from fundamental research to practical use, it is important to take into account the antioxidant, hydrolytic and thermal stability of the materials being developed," said Dmitry Lyubov, a project participant, Candidate of Chemical Sciences, senior researcher, Deputy head of the Laboratory of Metal Complex Catalysis at the Razuvaev Institute of Organometallic Chemistry of the Russian Academy of Sciences.
Miniaturization of electronics
The high density of information recording inherent in molecular magnets makes it possible to bring the miniaturization of electronic devices to a new level, which can be called one of the key criteria for the development of nanoelectronics, Anton Muravyev, Candidate of Chemical Sciences, associate Professor at the ITMO Scientific and Educational Center for Infochemistry, told Izvestia. According to him, the obtained indicators of temperature and oxidation stability are especially valuable, since it is these parameters that most often limit the widespread use of molecular magnets in the creation of functional materials.
"The high temperature and oxidation stability of erbium, dysprosium and terbium complexes (up to 290 °C), along with the demonstration of the properties of molecular magnets, attracts undoubted interest from the point of view of using these materials as devices for recording, storing and transmitting information in extreme temperature conditions and at high humidity levels," the expert said.
This study is extremely important because it is the first time that organometallic compounds of rare earth metals that are stable in the air environment have been obtained, which opens the way to their practical application, said Mikhail Gorshenkov, Associate Professor of the Department of Physical Materials Science at NUST MISIS. The discovery of magnetic properties in some complexes looks particularly promising, which can become the basis for creating ultra-high-density information carriers, the expert said. Using individual molecules as memory cells will make it possible to create data storage devices with incredible write density, orders of magnitude higher than modern hard drives and flash memory.
— In addition, molecular magnets based on rare earth elements can be considered promising candidates for creating qubits, the basic elements of quantum computers. Their stability at room temperature is a critical step in this direction. Another application is the creation of functional materials and sensors. These stable complexes can be used for spraying thin films or creating composites with specified magnetic properties. Such materials can be used in pintronics, microelectronics and as highly sensitive magnetic sensors," the scientist said.
Researchers from the Frumkin Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences (Moscow), the Kurnakov Institute of General and Inorganic Chemistry (Moscow), the University of Montpellier (France) and Maastricht University (the Netherlands) participated in the study.
The results of the study, supported by a grant from the Russian Science Foundation (RSF), are published in the journal Organic Chemistry Frontiers.
Переведено сервисом «Яндекс Переводчик»





